October 10, 2025
Understanding PV-IT: The Digital Core of Modern Drug Safety
Explore how Pharmacovigilance Information Technology (PV-IT) integrates data, compliance, and technology to strengthen global drug safety systems.
Pharmacovigilance is the science of ensuring that every medicine remains safe for the people who use it. Yet, in a world where data flows faster than ever, safety is not managed solely by scientists or regulators. It is sustained by systems that capture, store, and interpret millions of data points with precision. This is where Pharmacovigilance Information Technology (PV-IT) becomes the digital backbone of modern drug safety.
PV-IT combines the disciplines of data science, regulatory compliance, and enterprise technology to create an environment where patient safety can be monitored at scale. It connects the analytical power of machines with the ethical responsibility of human oversight. Within every global pharmacovigilance system, PV-IT ensures that information moves securely, accurately, and compliantly from one stage to the next.
What is PV-IT?
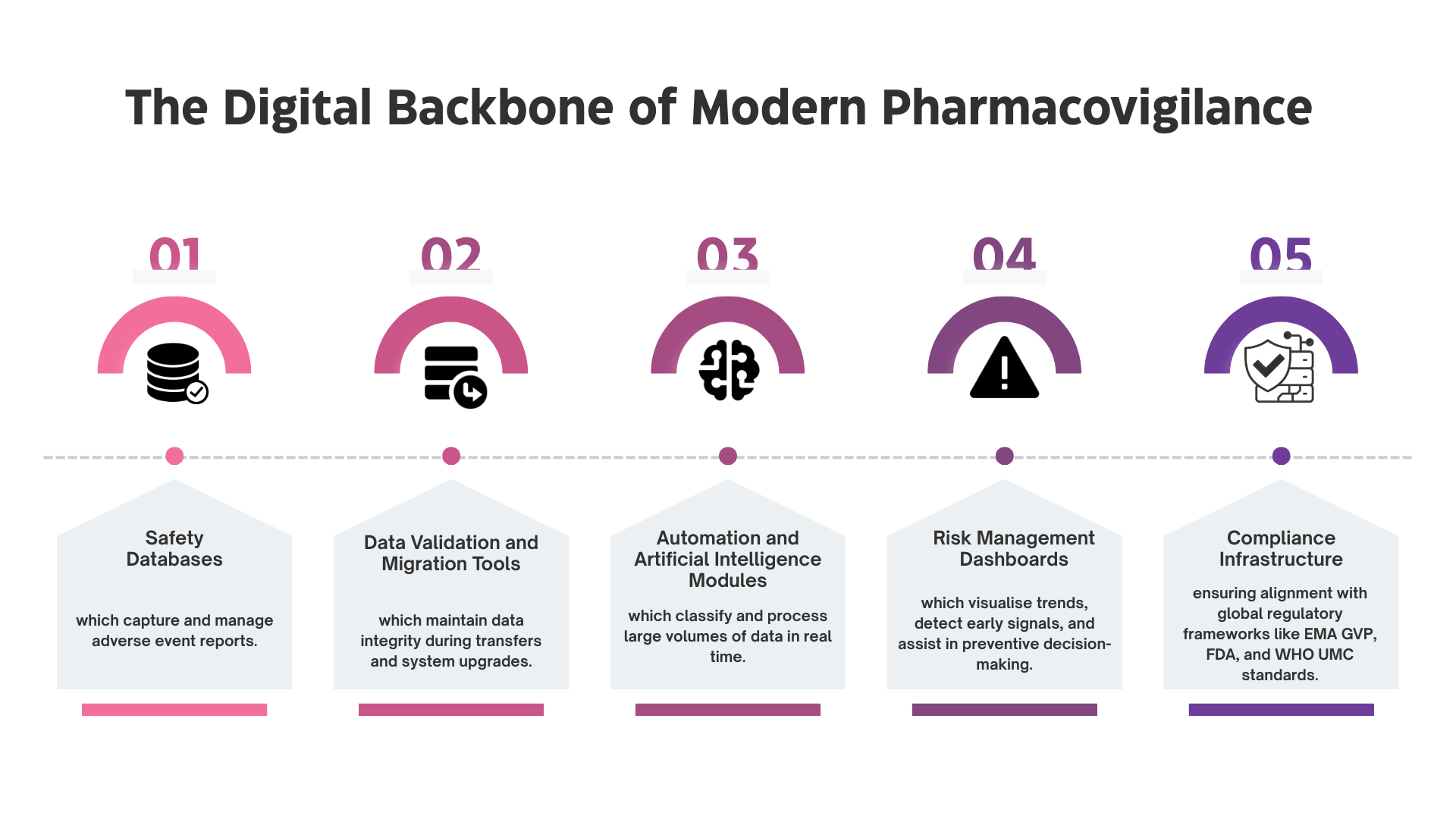
PV-IT is the information technology framework that powers pharmacovigilance. It includes the software, hardware, workflows, and validation processes that enable the collection, management, and reporting of adverse event data. Without PV-IT, safety reporting would remain fragmented and prone to errors.
Together, these elements form the technology-driven foundation that enables pharmaceutical companies to meet the growing demands for global safety reporting.
The Strategic Importance of PV-IT
Our PV-IT consulting and implementation frameworks align with EMA, FDA, and WHO standards to ensure clients achieve full regulatory readiness
In the life sciences industry, compliance and credibility depend on the quality of safety data. PV-IT safeguards that quality. It automates repetitive manual tasks, reduces human error, and provides consistent data across all geographies and regulatory authorities
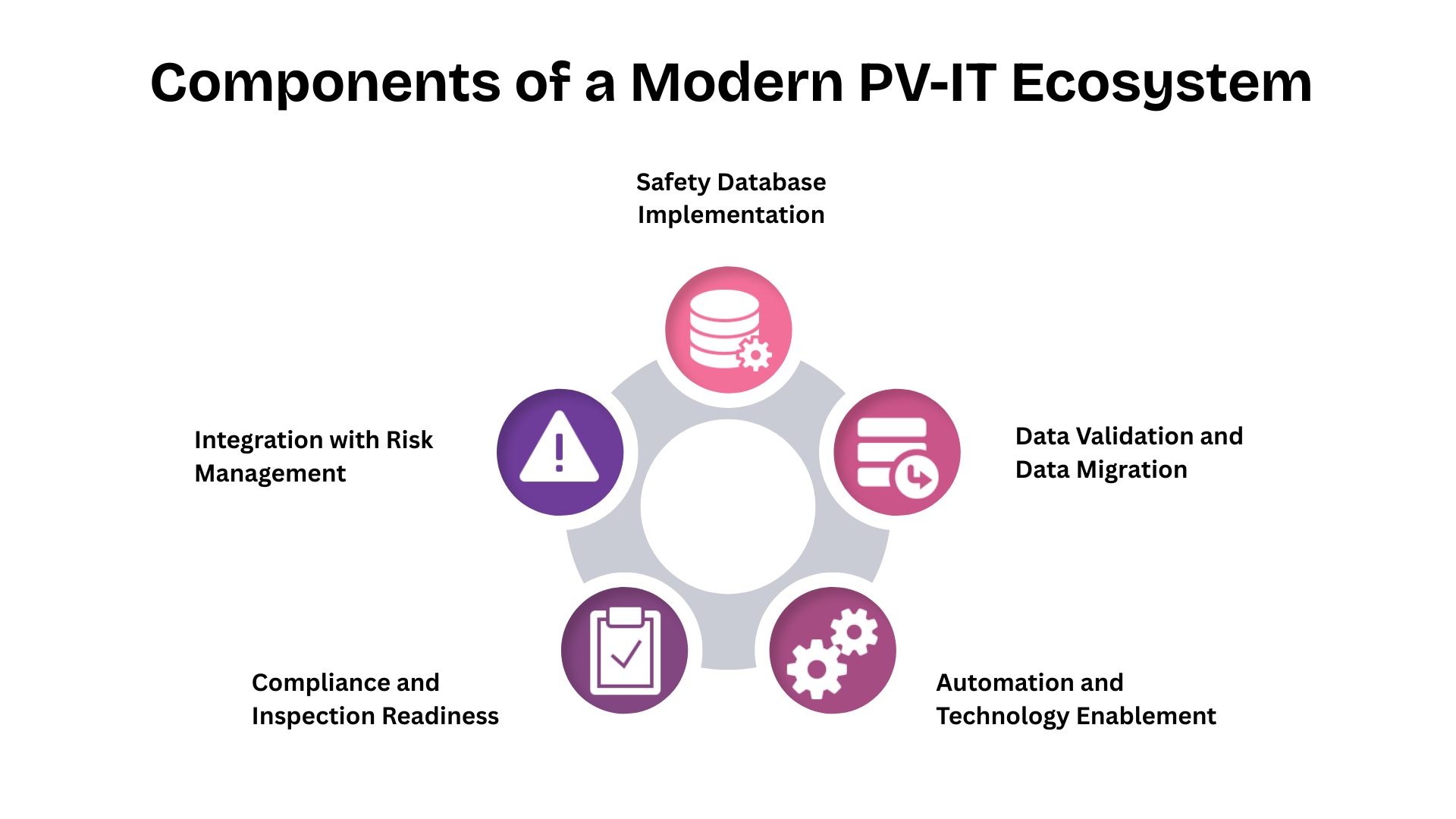
1. Safety Database Implementation
The safety database is the nucleus of any PV-IT environment. It houses every adverse event report, product complaint, and literature reference. Fidelity’s Safety Database Implementation solutions ensure that databases are validated, configurable, and aligned with global reporting systems such as EudraVigilance and FAERS.
Related Reading: Safety Database Implementation Services
2. Data Validation and Data Migration
Data accuracy defines the reliability of a safety system. Fidelity’s Data Validation and Data Migration services guarantee that all data transferred between legacy and modern systems remains complete, compliant, and audit-ready.
Learn more in our next article on Data Validation in Pharmacovigilance.
3. Automation and Technology Enablement
Automation transforms pharmacovigilance from reactive reporting to proactive intelligence. PV-IT integrates AI-based case triage, optical character recognition, and natural language processing to classify and analyse safety data efficiently. Fidelity’s Custom PV Software Development capabilities allow organisations to automate without compromising regulatory oversight.
Explore more in Custom Software Development for PV.
4. Compliance and Inspection Readiness
Every PV system must withstand global regulatory audits. PV-IT ensures that documentation, audit trails, and case histories are maintained in a compliant format. Fidelity’s Compliance and Inspection Readiness frameworks prepare organisations for inspections from agencies such as EMA, FDA, and MHRA.
See details in Compliance and Inspection Readiness Solutions.
5. Integration with Risk Management
Effective pharmacovigilance extends beyond reporting. PV-IT supports Risk Management Plans (RMPs) and Risk Evaluation and Mitigation Strategies (REMS) by integrating real-time safety monitoring tools and dashboards. Through analytics and automation, organisations can identify trends before they become compliance issues.
The Human Dimension of PV-IT
While PV-IT is powered by technology, its success depends on people. Regulatory experts, data scientists, and safety specialists work together to ensure that each system reflects the operational realities of pharmacovigilance. Fidelity builds systems that are not only compliant but intuitive, allowing users to navigate complex safety landscapes with confidence.
Organisational design and training are key aspects of successful PV-IT adoption. Global teams must understand data flow, system validation, and regulatory requirements. Fidelity provides targeted training and change-management programs to harmonise people, process, and performance across all safety functions.
Challenges PV-IT Solves
Modern pharmacovigilance faces several recurring challenges:
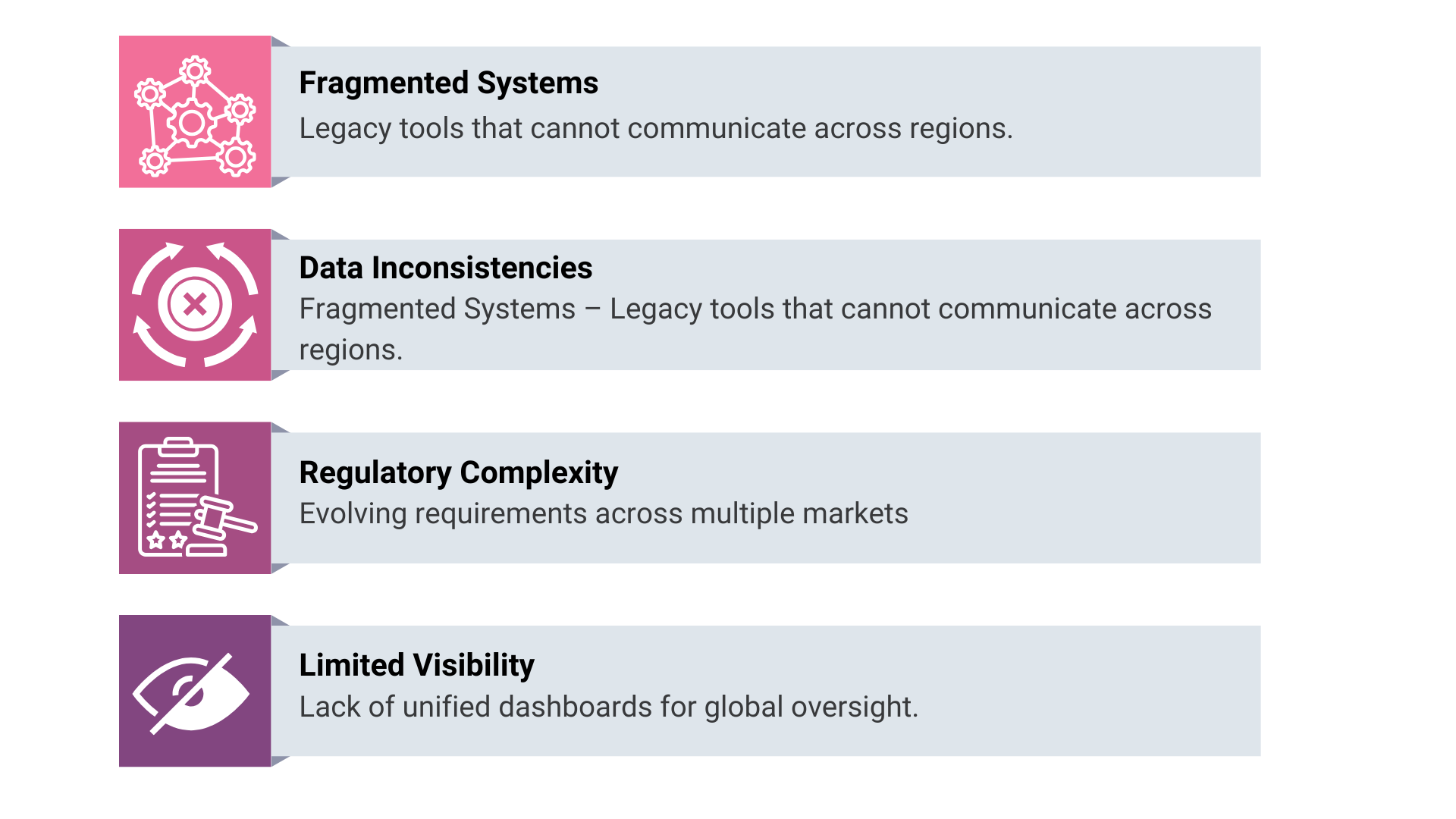
Through a cohesive PV-IT framework, these challenges are transformed into opportunities for operational excellence. Systems become interoperable, data becomes standardised, and compliance becomes continuous rather than episodic.
Building on our experience supporting global pharmacovigilance operations, Fidelity continues to shape the evolution of PV-IT with scalable, compliant, and future-ready platforms.
The Future of PV-IT
The next evolution of pharmacovigilance will be defined by AI integration, predictive analytics, and global collaboration. PV-IT will shift from processing reports to forecasting risks. Machine learning will detect safety signals before they escalate, and cloud-based platforms will enable synchronised oversight across continents.
Fidelity’s commitment is to create PV-IT environments that grow with these innovations. By combining technology enablement with regulatory intelligence, Fidelity ensures that pharmacovigilance remains not only compliant but visionary.
Discover how emerging technologies are redefining safety in our upcoming article on Technology Enablement in PV.
Conclusion
PV-IT is no longer an optional layer of support; it is the foundation of every effective pharmacovigilance system. It connects people, processes, and technology in a unified framework that transforms safety data into actionable intelligence.
At Fidelity Health Services, PV-IT is not just about infrastructure. It is about building trust through technology, ensuring that every report, every case, and every insight contributes to the global mission of safer healthcare.
With proven expertise across safety systems, validation, and digital transformation, Fidelity partners with clients to make every pharmacovigilance operation smarter, faster, and regulatory-ready.
For a deeper look into our integrated PV-IT solutions, visit our pages on Data Validation, Data Migration, Safety Database Implementation, and Custom PV Software Development.
