October 31, 2025
Safety System Modernisation: Building the Infrastructure of Compliance
Explore how modernisation of safety systems transforms pharmacovigilance from reactive reporting to proactive compliance. Learn how Fidelity builds scalable, inspection-ready infrastructures
Modern pharmacovigilance depends on more than accurate case processing or regulatory diligence. It depends on the strength of the systems that manage data, automate reporting, and support decision-making. As regulations evolve and data volumes expand, traditional safety infrastructures often struggle to keep pace. This creates risk, inefficiency, and potential non-compliance.
Safety System Modernisation is the process of transforming legacy pharmacovigilance systems into scalable, compliant, and intelligent infrastructures. It ensures that every safety operation is supported by reliable technology, validated processes, and transparent data flow. For global life sciences organisations, modernisation is no longer an upgrade; it is a requirement for sustained compliance.
What is PV-IT?
PV-IT is the information technology framework that powers pharmacovigilance. It includes the software, hardware, workflows, and validation processes that enable the collection, management, and reporting of adverse event data. Without PV-IT, safety reporting would remain fragmented and prone to errors.
Together, these elements form the technology-driven foundation that enables pharmaceutical companies to meet the growing demands for global safety reporting.
What is Safety System Modernisation?
In pharmacovigilance, modernisation refers to the systematic transformation of technology, processes, and workflows that support the capture, management, and analysis of adverse event data. It integrates regulatory expectations with technical efficiency, ensuring that organisations remain audit-ready and data-accurate at all times.
A modern safety system does more than collect information. It standardises it, validates it, and makes it accessible for timely decision-making. Through automation and advanced analytics, modernised systems reduce manual dependencies and improve the accuracy of global safety submissions.
Safety System Modernisation, when executed strategically, results in a unified architecture that connects databases, integrates automation, and ensures compliance with global frameworks such as the EMA Good Pharmacovigilance Practices, FDA MedWatch Program, and WHO UMC standards.
Why Legacy Systems Fail?
Many pharmacovigilance teams continue to rely on systems that were designed before modern compliance requirements existed. These legacy systems often lack the flexibility and integration capacity necessary to handle the complexity of current safety data workflows.
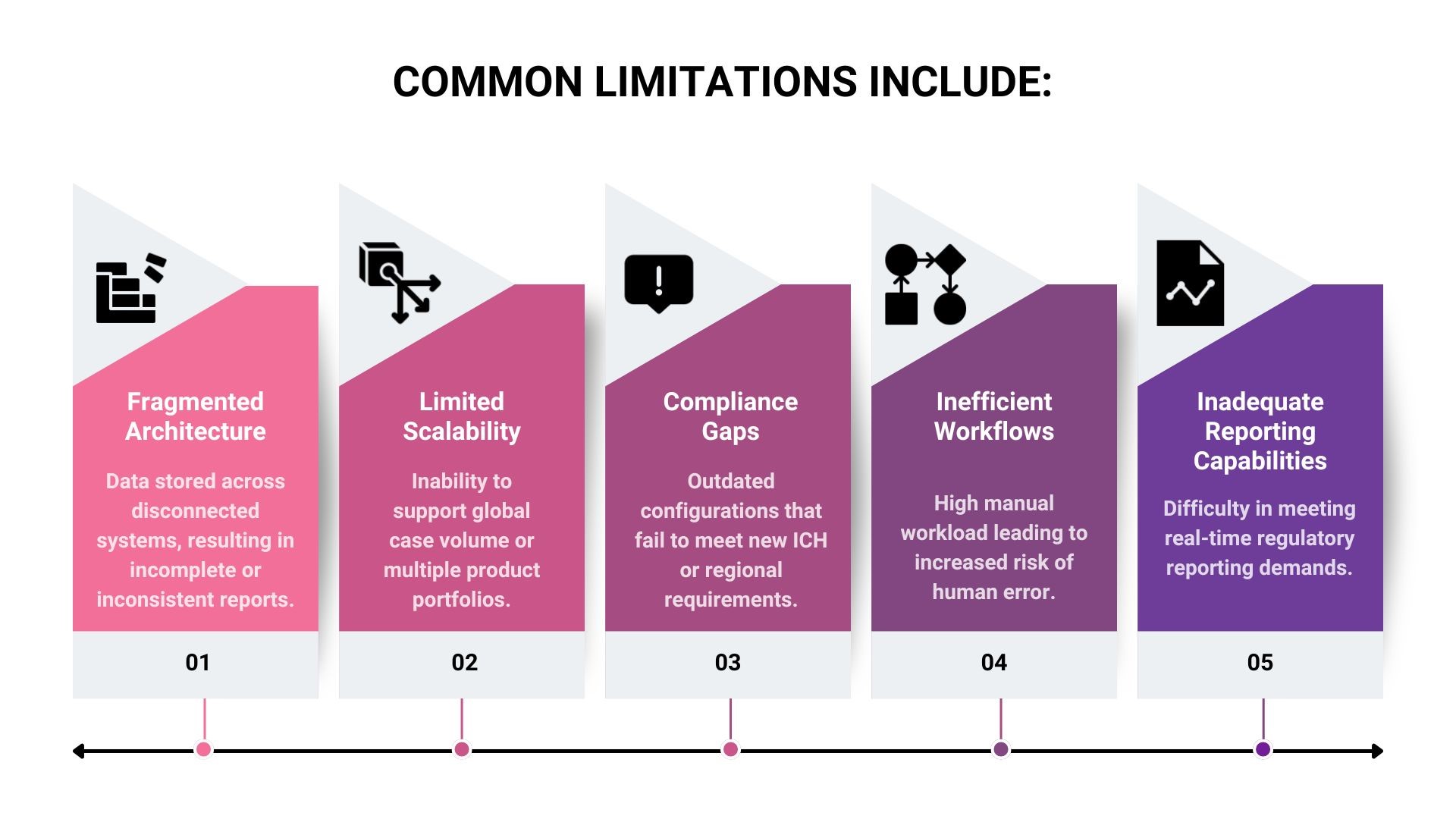
These challenges not only slow operations but can also expose organisations to compliance risks, audit failures, and data inaccuracies.
The Architecture of a Modern Safety System
A modern pharmacovigilance safety system integrates all safety-related functions into a single, validated environment. This architecture ensures seamless data movement, visibility across operations, and readiness for inspection at any time.

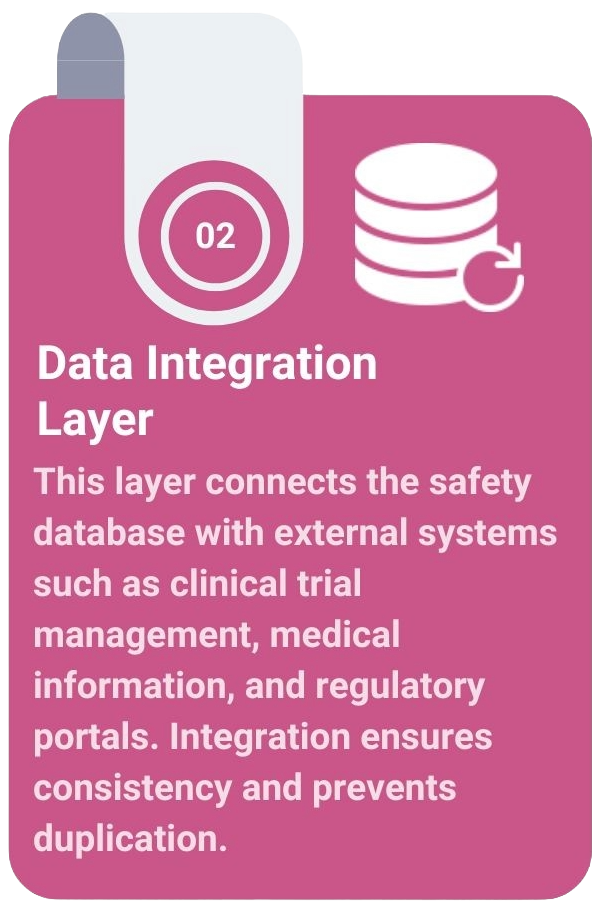



PV-IT: The Engine Behind Modernisation
Safety system modernisation is a direct function of PV-IT. It involves a combination of data validation, migration, automation, and compliance monitoring that together define operational integrity.
Through Pharmacovigilance Information Technology, Fidelity enables organisations to unify their safety data ecosystem. Each process case entry, assessment, signal detection, and regulatory reporting is supported by technology that ensures quality and compliance.
Fidelity’s PV-IT implementation practice ensures that safety modernisation efforts align with both regulatory expectations and client operational realities.
Related Reading: Understanding PV-IT: The Digital Core of Modern Drug Safety
Fidelity’s Approach to Safety System Modernisation
Fidelity Health Services modernises pharmacovigilance systems with precision and regulatory intelligence. Every modernisation project begins with a deep assessment of the existing infrastructure and ends with a fully validated, inspection-ready platform.
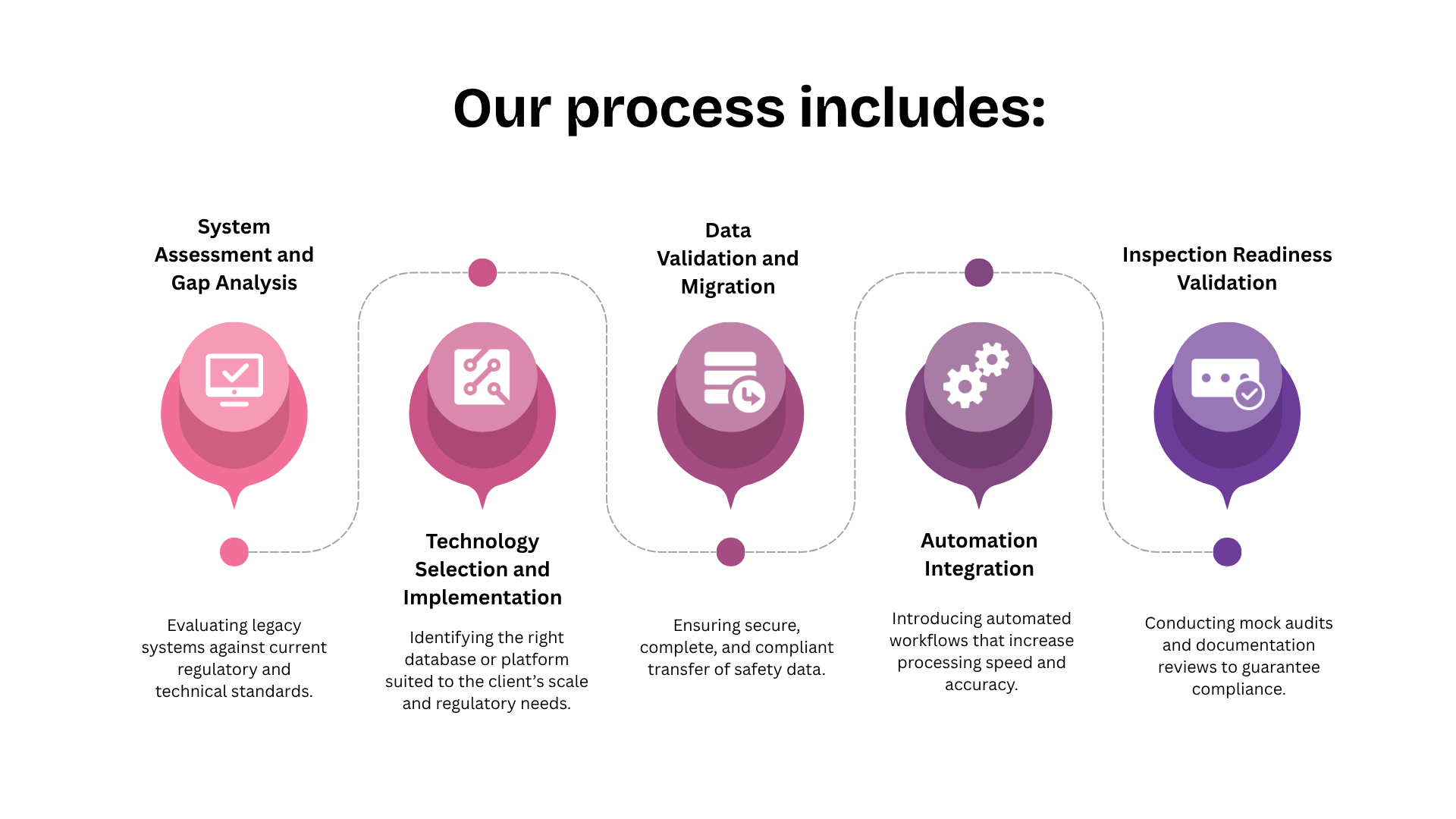
Explore more about our Data Validation and Migration Services and Compliance and Inspection Readiness Frameworks.
The Compliance Advantage
Modernisation strengthens compliance at every stage of pharmacovigilance. From data collection to reporting, each component of a modern system aligns with international regulations.
- EMA requires traceability, consistency, and submission readiness under the GVP guidelines.
- FDA emphasises data integrity and real-time submission capability through the FAERS system.
- WHO UMC promotes harmonisation of safety data across countries and systems.
A modernised PV-IT infrastructure ensures that these expectations are met continuously rather than reactively. It provides visibility, auditability, and operational harmony across global teams.
Global Alignment through Operational Harmonisation

Safety System Modernisation does not only involve technology. It requires alignment of people and processes. Fidelity’s Operational Harmonisation approach integrates standard operating procedures, training programs, and cross-functional coordination to ensure consistent performance across all affiliates.
This creates a unified structure where every stakeholder safety specialist, IT administrator, or regulatory lead works within the same compliant framework.
Our teams work closely with clients to align regional PV operations under a common digital governance structure, enabling seamless collaboration and continuous compliance.
Learn more in our upcoming article on Operational Harmonisation in Pharmacovigilance.
The Future of Safety Systems
As pharmacovigilance becomes more data-driven, safety systems are moving toward predictive automation. Artificial intelligence will not only classify reports but will also forecast potential risks using historical data. Cloud-based infrastructures will enable real-time collaboration between affiliates and regulators.
Fidelity’s PV-IT roadmap focuses on enabling these transitions responsibly. Every innovation is integrated with validation, security, and compliance to maintain the trust of patients and regulators alike.
Conclusion
Safety System Modernisation is more than a technological upgrade; it is a structural transformation that defines the future of pharmacovigilance. It builds a foundation where compliance, efficiency, and scalability coexist.
At Fidelity Health Services, modernisation means creating inspection-ready systems that evolve with regulatory change and technological advancement. It is the infrastructure through which safety data becomes knowledge and compliance becomes confidence.
To explore our complete range of PV-IT solutions, visit our pages on Data Migration, Data Validation, and Custom PV Software Development.
