
December 18, 2025
Operational Harmonisation: Aligning People, Process, and Performance in Pharmacovigilance
Learn how aligning people, process, and performance strengthens global pharmacovigilance systems. Explore Fidelity’s approach to achieving operational harmony across PV-IT environments for patient safety and regulatory trust.

Pharmacovigilance is no longer defined by regulatory compliance alone. In a world of constant data exchange and evolving technologies, the efficiency of safety systems depends on one core principle: operational harmony. When people, processes, and performance align seamlessly, pharmacovigilance moves from reactive case management to proactive safety intelligence.
In practice, most organisations possess advanced technology but still face inefficiencies caused by disconnected teams or inconsistent workflows. Operational harmonisation bridges that gap. It ensures that every activity, from data entry to regulatory submission, functions as part of a synchronised system. Within Fidelity’s PV-IT ecosystem, harmonisation is not a project; it is an ongoing discipline that ensures accuracy, compliance, and confidence across the organisation.
The Meaning of Operational Harmonisation in PV-IT
Operational harmonisation refers to the process of aligning human resources, standard procedures, and technological systems under a unified framework. In pharmacovigilance, it means creating a consistent approach to data handling, communication, and compliance across global affiliates.
A harmonised PV operation guarantees that:
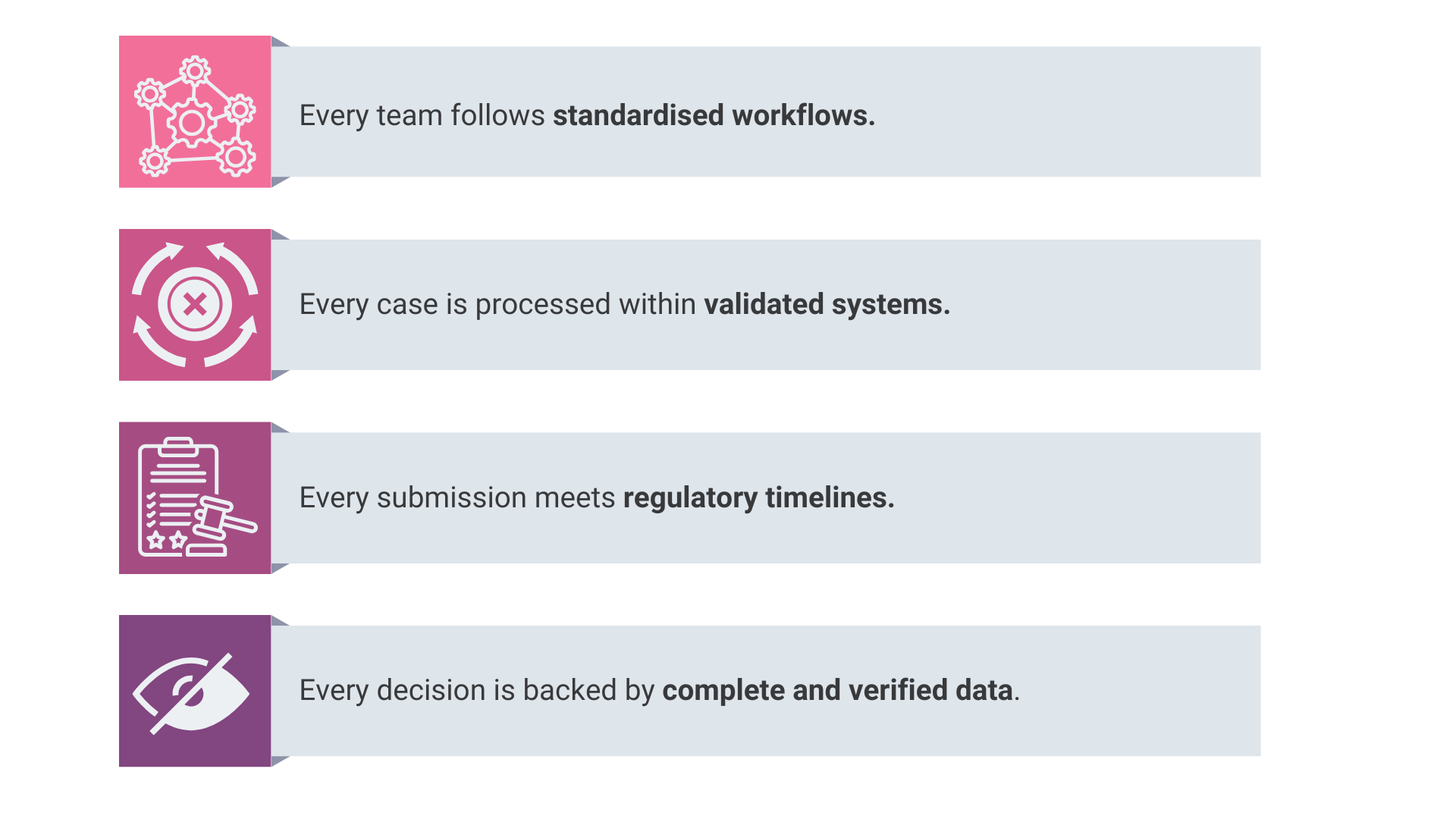
This alignment is critical as pharmacovigilance expands across continents and regulatory frameworks. It ensures that the organisation functions as one entity even when distributed across multiple regions and systems.
The Human Factor in Harmonisation
Technology alone cannot achieve harmonisation. The success of PV-IT systems depends on the people who use them.
Global teams must interpret safety data uniformly, understand system workflows, and apply standard operating procedures with precision. Fidelity emphasises the human dimension of pharmacovigilance by designing training programs, competency models, encouraging culture of compliance, and change management frameworks that build long-term consistency.
These initiatives cultivate a shared culture of safety, one where quality is not an obligation but a collective habit.
Related Reading: Data Validation and Migration: The Hidden Engines of Trust in Pharmacovigilance.
Processes that Create Consistency
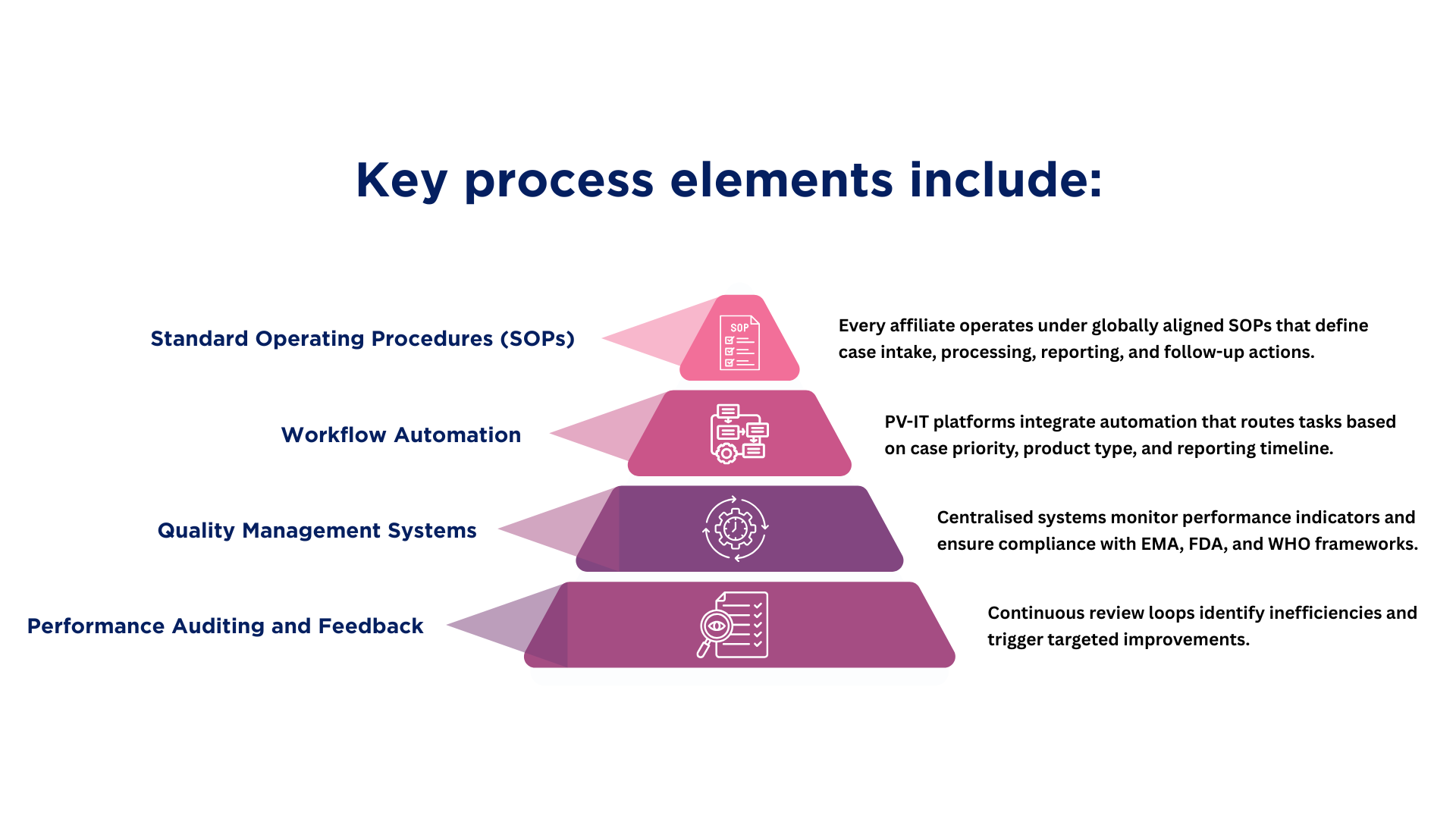
When these processes operate within validated systems, pharmacovigilance achieves not only consistency but also measurable efficiency.
Technology as the Enabler of Harmony
While harmonisation begins with people and process, it is sustained by technology. Modern PV-IT environments serve as the digital foundation that connects every layer of operations.
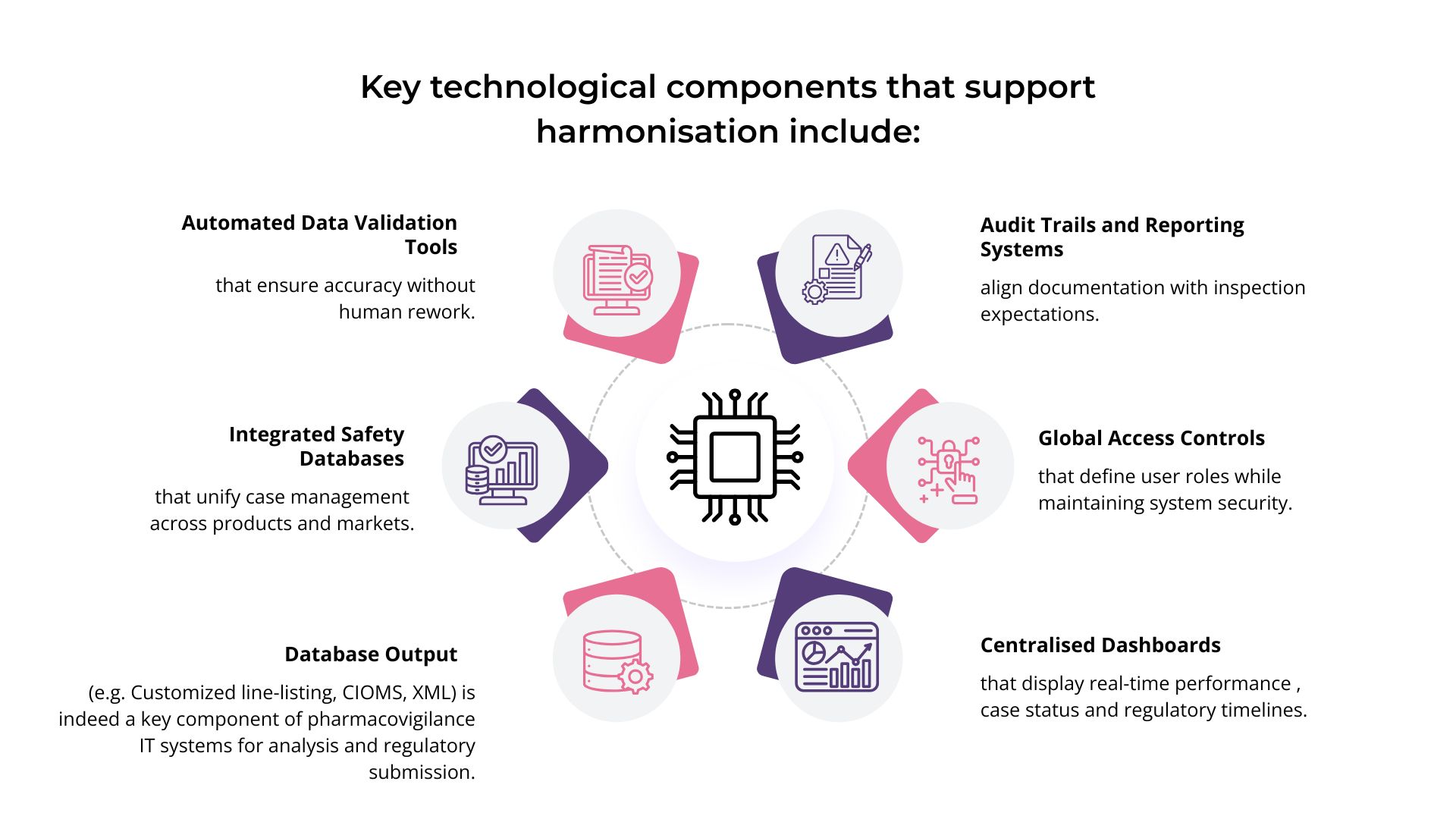
By embedding these tools into daily workflows, organisations transform compliance into an effortless outcome rather than a recurring challenge.
Explore related topics in Safety System Modernisation, Building the Infrastructure of Compliance.
The Role of Leadership and Culture
Operational harmony depends heavily on leadership commitment. Executives must champion collaboration and establish accountability across departments. Fidelity’s advisory teams often guide organisations through governance structure development, defining clear ownership models for global PV processes and promoting patient safety as a core organisational value.
A strong governance culture ensures that every regional team understands its responsibilities within the global framework. Decision-making becomes faster, reporting becomes transparent, and performance becomes measurable. This cultural alignment turns pharmacovigilance into a continuous cycle of learning and improvement.
Regulatory Alignment and Global Coordination
The global nature of pharmacovigilance demands that operations align with multiple regulators. Harmonised systems allow organisations to meet different regional requirements without redundancy.
For instance, the EMA Good Pharmacovigilance Practices framework requires defined responsibilities across partners and affiliates, while the FDA 21 CFR Part 11guidance emphasises traceability and audit readiness.
Through PV-IT integration, Fidelity ensures that clients maintain alignment with both, enabling smooth coordination between regional teams and the global oversight unit.
Case Insight: The Cost of Fragmentation
In one industry case, a multinational organisation operating across four regions used different safety databases with varying SOPs. The result was delayed submissions, inconsistent reports, and repeated audit/inspection findings. When the company adopted a harmonised PV-IT framework, integrating automation and centralised dashboards, reporting timelines improved by thirty per cent, and audit observations dropped to zero.
This case illustrates that harmonisation is not a conceptual ideal but a measurable operational advantage.
Challenges in Achieving Harmonisation Common barriers include:
The global nature of pharmacovigilance demands that operations align with multiple regulators. Harmonised systems allow organisations to meet different regional requirements without redundancy.
- Resistance to process change.
- Lack of global SOP alignment.
- Inconsistent data taxonomies.
- Limited training across affiliates.
Fidelity addresses these challenges through a structured Operational Harmonisation Framework that includes:
- Global SOP Development and Standardisation.
- Cross-functional Workshops for process mapping and alignment.
- Cross-functional Workshops for process mapping and alignment.
- Ongoing Training and Change Management programs.
This framework transforms fragmented systems into unified networks that function under a single quality and compliance philosophy.
The Future of Harmonised Pharmacovigilance
Future-ready pharmacovigilance will depend on dynamic alignment rather than static procedures. As new regulatory requirements emerge, systems must adapt instantly without disrupting established workflows. Artificial intelligence and real-time analytics will play a pivotal role in maintaining global harmonisation, offering predictive insights into workload, performance, and compliance health.
Fidelity continues to invest in this evolution by developing PV-IT architectures that respond intelligently to change. In a harmonised ecosystem, people will focus on strategic safety decisions while technology manages consistency, traceability, and compliance.
Conclusion
Operational Harmonisation stands at the intersection of people, process, and technology. It transforms pharmacovigilance from a series of isolated tasks into a unified, intelligent operation capable of sustaining compliance and innovation at scale.
At Fidelity Health Services, harmonisation is a commitment to continuity. Every system we modernise, every process we validate, and every team we train contributes to a single purpose: building organisations where safety operations perform as one.
For more insights into integrated PV-IT systems, explore our related articles on Data Validation, Safety System Modernisation, and Risk Management in Pharmacovigilance.