
November 15, 2025
Data Validation and Migration: The Hidden Engines of Trust in Pharmacovigilance
Discover how accurate data validation and secure data migration sustain global drug safety systems. Learn how Fidelity builds reliability and compliance through PV-IT excellence.
Behind every compliant pharmacovigilance operation lies one constant truth: accuracy creates trust. Each adverse event, medical inquiry, and regulatory report must be stored and transmitted without error. If the data is flawed, the entire chain of patient safety can collapse. This is why data validation and data migration stand at the core of modern Pharmacovigilance Information Technology (PV-IT).
As companies upgrade to new safety databases or automate reporting, they must ensure that information integrity is never compromised. Validation and migration provide that assurance. They safeguard data through every transformation, guaranteeing that what regulators review tomorrow is identical to what the system captured today.

Understanding Data Validation and Migration in PV
Within PV-IT, data validation refers to the process of verifying that all safety data entered or transferred into a system meets defined accuracy and completeness standards. Data migration involves moving that validated information from one database or platform to another while maintaining its fidelity.
Together, they serve as the digital quality framework for pharmacovigilance. Validation ensures the data is correct; migration ensures it remains intact when systems evolve. Both are required by global regulations such as the EMA Good Pharmacovigilance Practices, FDA 21 CFR Part 11, and ICH E6(R3).
Our team supports clients through every phase of PV data migration — from validation and system testing to inspection-ready documentation.

Why Data Integrity Matters
Regulators expect safety data to be accurate, traceable, and reproducible. Any deviation can delay submissions, trigger inspections, or undermine the credibility of a company’s pharmacovigilance system. Data integrity is not just a compliance requirement; it is the foundation of patient trust.
Without proper validation and migration frameworks, organisations risk:
- Data loss during system transitions
- Duplicated or corrupted records
- Inaccurate safety signal detection
- Regulatory non-compliance during audits
In a discipline where every report can influence clinical decisions or product approvals, even minor data discrepancies can have global consequences.
Data Validation: Building Accuracy and Auditability
Data validation is the systematic verification that ensures each data point in a safety system is accurate, consistent, and compliant with regulatory expectations.
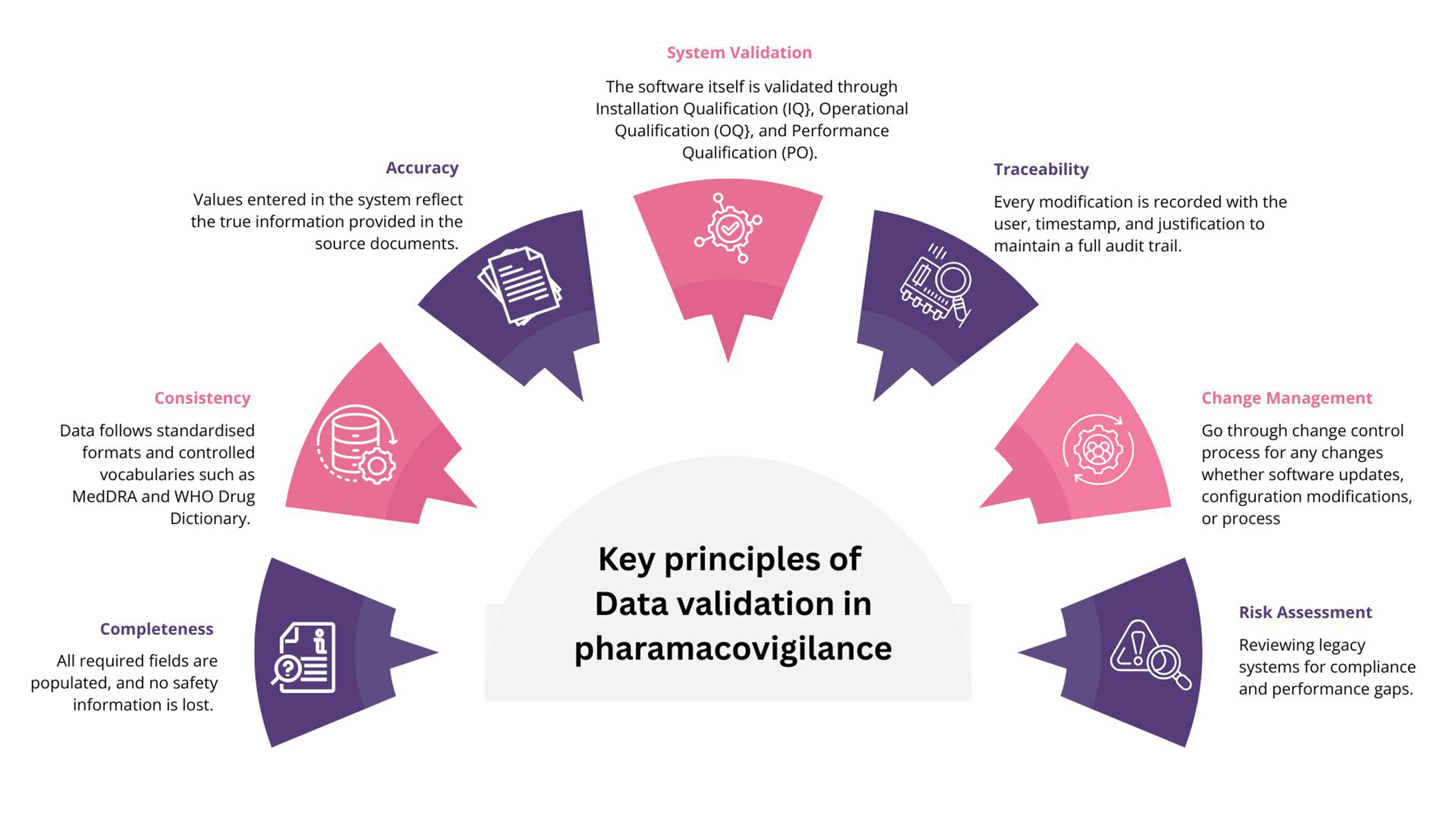
Validated systems demonstrate to regulators that data is both technically and procedurally trustworthy.
Related Reading: Safety System Modernisation Building the Infrastructure of Compliance
Data Migration: Moving Safety Data with Precision
Fidelity Health Services has made significant investments in creating a proprietary Data Migration & Integration Framework designed to streamline and expedite complex migration processes.
This migration process is essential for maintaining the integrity, accessibility, and compliance of safety data during system upgrades, platform changes, or integrations. A properly executed data migration can help pharmaceutical companies avoid expensive compliance issues, including both financial and legal penalties.
The Fidelity Health Services team is expertly equipped to manage all your data migration requirements. We have successfully overseen migrations ranging from hundreds to millions of cases, handling diverse business situations—whether it is transitioning to a new system, acquiring new products, changing service providers, or navigating mergers and acquisitions.
Migration is not merely the transfer of information; it is the preservation of history. Every case record represents evidence of a patient experience. During upgrades or vendor transitions, that evidence must be protected.
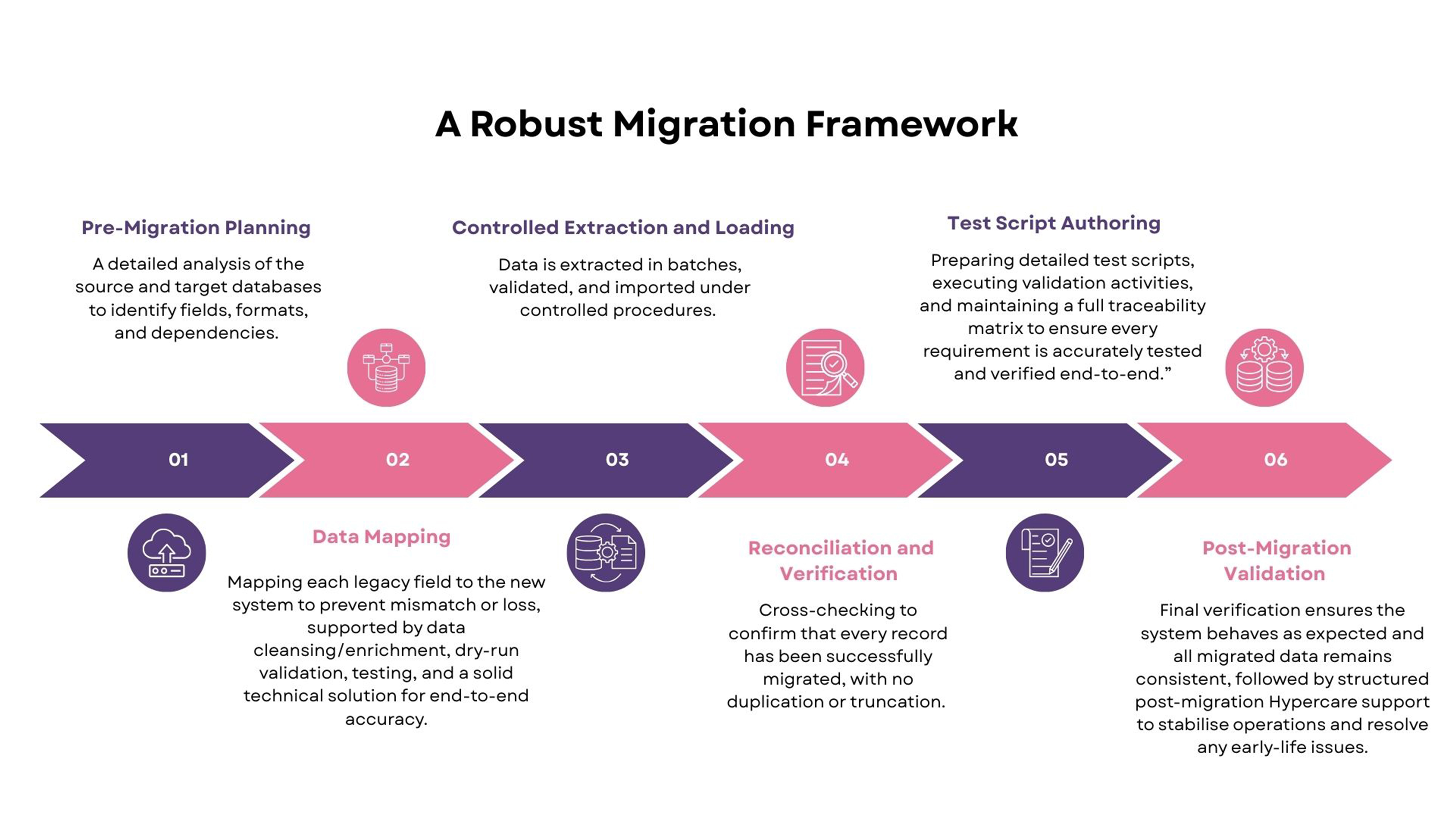
Why Migration Test Matters
- To avoid any disruption/inconvenience.
- To ensure that user can continue to use all the features of the application.
- To ensure compatibility of the upgraded application with hardware and software.
- To ensure the existing functionalities works seamlessly.
- Critical defects need to be identified & fixed during testing.
- To ensure no performance degradation post migration.
Through these steps, Fidelity enables seamless transitions between platforms while maintaining continuous regulatory compliance
Our safety system data migration solutions are designed to meet a wide range of needs, including:
- Migrations involving small to large data volumes
- E2B-based migrations.
- Transfers from one or multiple source systems to one or multiple target systems.
- A combination of E2B and database migrations.
- Data extractions.
- Case data updates.
- Data cleanup tasks
Fidelity’s PV-IT Approach
At Fidelity Health Services, data validation and migration are not separate activities; they are integral to every PV-IT engagement. Each project begins with an evaluation of existing systems, identifying gaps and risks before any migration begins

Fidelity’s structured methodology includes :
- Requirement Management - Gathering, documenting, and approving all system requirements
- System Audit and Risk Assessment - Reviewing legacy systems for compliance and performance gaps
- Test Case Generation - Detailed test cases are created to verify that each system requirement is met
- Trace Matrix Generation - A Traceability Matrix links requirements to design specifications, risks, and test cases. It ensures that every requirement is covered by at least one test and that no critical functionality is overlooked.
- Validation Protocol Development – Establishing IQ, OQ, and PQ standards aligned with regulatory expectations and documenting the actual results.
- Secure Migration Execution – Implementing verified scripts and automated controls to ensure data accuracy.
- Reconciliation and Documentation – Creating detailed reports to demonstrate traceability and completeness.
- Regulatory Submission Support – Preparing audit-ready evidence for inspections by EMA, FDA, or MHRA.
This integrated approach reduces downtime, eliminates data inconsistencies, and delivers
full transparency to regulators.
Explore related services on Data Validation and Migration and Compliance, and
Inspection Readiness.
Challenges and Fidelity’s Solutions:
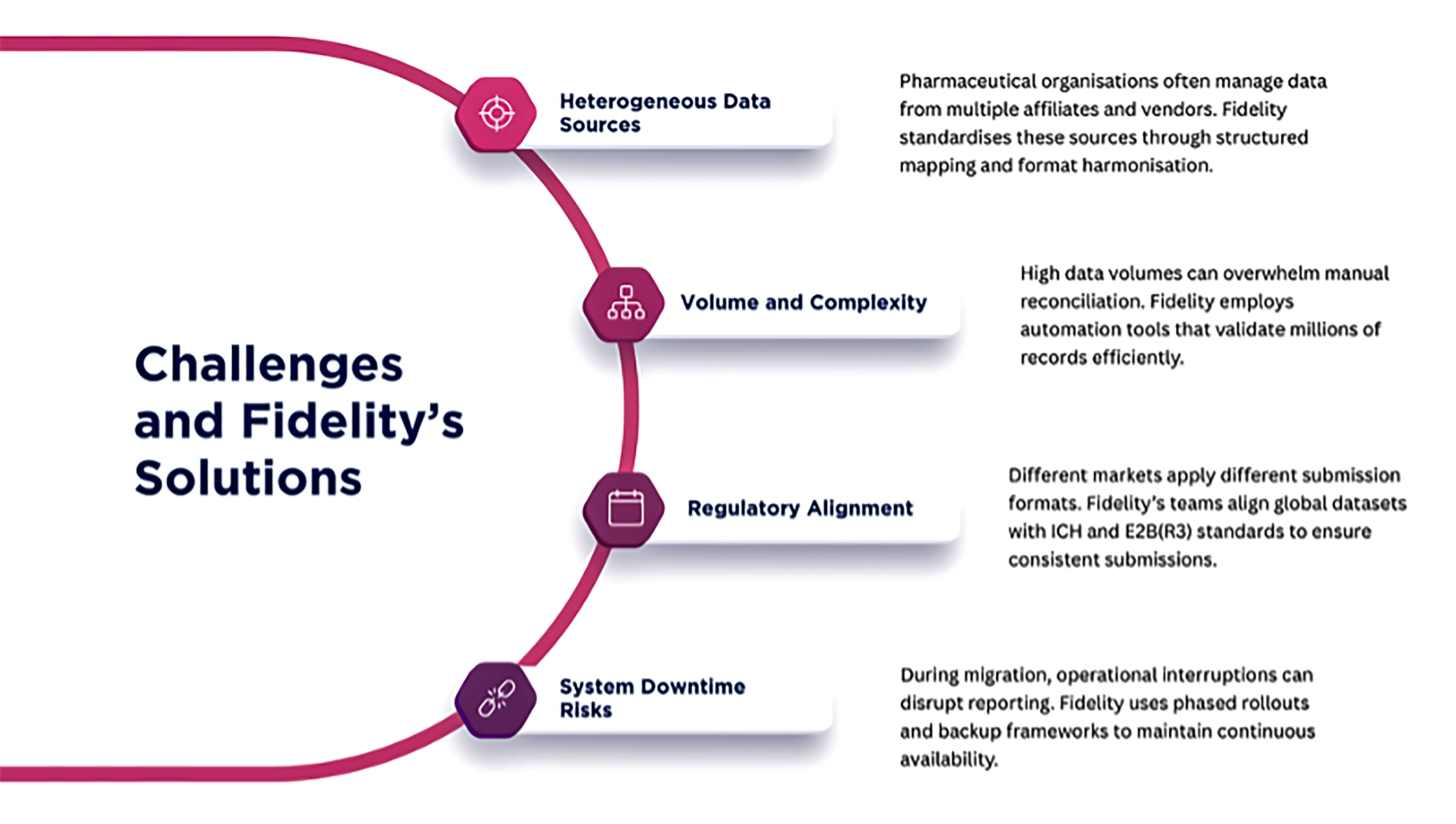
Through these measures, organisations move from uncertainty to operational confidence.
Global Regulatory Context
Global authorities emphasise validated systems and documented migration activities as essential components of pharmacovigilance compliance.

Fidelity’s PV-IT methodology is aligned with these standards, ensuring that modernisation meets both technical and regulatory benchmarks.
Interconnection within PV-IT
Data validation and migration connect directly with all other pillars of PV-IT. Without validated data, Safety System Modernisation cannot achieve reliability. Without secure migration, Compliance and Inspection Readiness cannot be proven
Related Reading :
- Understanding PV-IT: The Digital Core of Modern Drug Safety
- Safety System Modernisation: Building the Infrastructure of Compliance
- Compliance and Inspection Readiness in Pharmacovigilance - This interlinking structure forms the foundation of Fidelity’s integrated PV-IT services.
The Future of Data Management in Pharmacovigilance
The next generation of PV-IT will use continuous validation, automated reconciliation, and machine-assisted quality checks. Cloud-based environments will maintain compliance dynamically, eliminating the need for periodic manual revalidation. Artificial intelligence will flag anomalies before they create compliance issues.
Fidelity continues to invest in such innovations, ensuring that every system implemented today remains compliant tomorrow. The future of pharmacovigilance data management will not depend on human vigilance alone but on intelligent systems designed with regulatory foresight.
Fidelity’s PV-IT experts continue to integrate automation, AI, and quality frameworks to ensure systems remain compliant, intelligent, and adaptive.
Conclusion
Data validation and migration may remain invisible to most stakeholders, but they form the silent engines that drive pharmacovigilance integrity. They ensure that the information supporting every medical decision is authentic, complete, and verifiable.
At Fidelity Health Services, these processes are executed with precision, transparency, and regulatory intelligence. By embedding validation and migration within every PV-IT solution, Fidelity transforms data into a trusted asset that protects patients, empowers compliance, and upholds the credibility of global healthcare.
To continue exploring how PV-IT shapes modern pharmacovigilance, visit our articles on Safety System Modernisation and Operational Harmonisation.