Why smart case processing in pharmacovigilance needs automation more than ever before
Pharmaceutical and biopharma companies are required by regulatory compulsions to implement a pharmacovigilance/drug safety surveillance programme and monitor the safety profiles of their marketed products during the complete product lifecycle.
Companies have increasingly started focusing on the reorganisation of drug safety and risk management programs to facilitate proactive identification and prediction of safety signals and benefit-risk evaluation for marketed medicines, along with amalgamating data sets across all the stakeholders (pharmaceutical companies, regulatory authorities, patients and healthcare providers) to empower complete transparency, sharing and partnership.
Numerous safety databases available in the market like Oracle Argus, ARIS-G etc. are used by the industry to process and report adverse events (AEs) to local regulatory authorities.
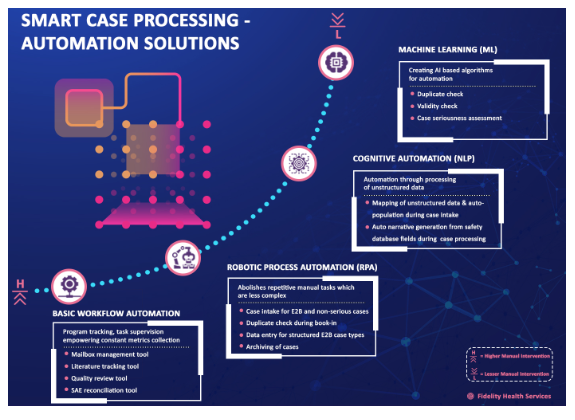
Need for transformation to smart case processing
With the evolving regulatory environment and increased regulatory scrutiny, increasing disease complexity and number of drugs getting approved, growing awareness with patients and providers about reporting of adverse events, social media connectivity resulting in a huge influx of data and source documents with multiple templates or formats, there is increasing need for pharmaceutical companies to deploy and maintain more complex PV systems and manage safety surveillance activities more meticulously and proficiently than ever.It's a need of the hour to revisit the traditional manual methods of case processing due to both the reduced supply of safety talent vis-à-vis demand and pressures on the companies to reduce the costs of manual case processing due to the increasing number of adverse events getting reported year on year.
As per the report published by Deloitte, pharmaceutical companies are allocating 40-85 % of PV budgets on case processing, and case volumes are increasing at a rate of 10-15 per cent per year.
As mentioned in the report, reducing the cost of case processing was the primary goal for 90% of respondents and survey respondents expected automation to deliver the cost savings of 30% per ICSR.